CONCEPT
Japan Clinical Trial Transformation (CTX) Research Society aims to enhance participation in global clinical trials and resolve drug lag / drug loss issues by transforming the clinical trial environment in Japan through multi stakeholder engagement.
 PURPOSEEnhancing participation in global clinical trials by transforming the clinical trial environment in Japan through multi-stakeholder engagement.
PURPOSEEnhancing participation in global clinical trials by transforming the clinical trial environment in Japan through multi-stakeholder engagement. VISIONContributing to the advancement of pharmaceutical development in Japan through policy recommendations and initiatives that support the dissemination of CTX.
VISIONContributing to the advancement of pharmaceutical development in Japan through policy recommendations and initiatives that support the dissemination of CTX. MISSIONAdvancing the efficiency of clinical trials through CTX, while enhancing participation in global clinical trials, to deliver new therapeutic options to patients more quickly on a world level.
MISSIONAdvancing the efficiency of clinical trials through CTX, while enhancing participation in global clinical trials, to deliver new therapeutic options to patients more quickly on a world level.
Clinical Trial Transformation (CTX) is one of the means for Japan to remain "chosen for global clinical trials."
CTX is transforming the methodologies of clinical trials to conduct them more efficiently and effectively.
To align with international trends, promoting CTX and enhancing participation in global clinical trials are urgent priorities in Japan.
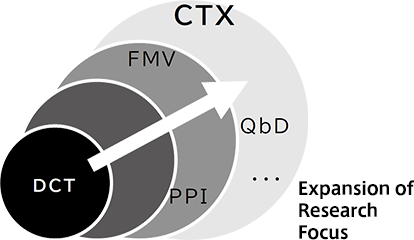
First, we focus on DCT as an initial topic for promoting CTX. DCT has gained momentum worldwide especially during the COVID-19 pandemic. In Japan, however, regulatory preparations are still in progress for implementation of DCT. It is essential for stakeholders to share a common understanding regarding the risks of falling behind in worldwide DCT adoption and concerns and uncertainties related to DCT implementation. Additionally, the creation of evidence and case studies of successful DCT implementation is crucial for widespread adoption.
Based on the above, we aim to foster a shared understanding among stakeholders on DCT, delve into the challenges of DCT adoption and dissemination, and propose the necessary government efforts (regulations, systems, support, etc.) to create an environment conducive to generating evidence and case studies.
- ※1 Decentralized Clinical Trials (DCT) are conducted remotely, allowing patients to participate from their homes or other remote locations without visiting a medical institution. They are also known as online trials or remote trials.
BACKGROUND
In recent years, the issue of drug lag/ drug loss*2 in Japan has once again become a concern, prompting a sense of urgency in government committees. One of the contributing factors is the low participation rate of Japan in global clinical trials (Japan's participation rate was 21.0% for 1,035 international collaborative trials conducted in 2018*3). To ensure access to new drugs, it is crucial for Japan to establish an environment for global clinical trials, in other words, to be "chosen for clinical trials." CTX is a key means to achieve this.
- ※2 Drug lag/ drug loss refer to the time lag or inability to use a drug in Japan that has already been approved and utilized in other countries.
- ※3 Office of Pharmaceutical Industry Research (July 2022), 「近年における国際共同治験の動向調査」政策研ニュースNo.66

PROSPECTS
First, we will focus on the recent topic of DCT and generate concrete achievements.
Then, we plan to gradually expand the scope of discussion in line with the objectives of our association to promote CTX in relevant areas. We will examine the significance of clinical trial participation in addressing drug lag/loss, ensuring equal opportunities for patients, and other aspects from the perspectives of DCT, QbD, FMV, and other methods.
- Issues in Japan's
Clinical Trial Environment - Transformaion to make clinical trials
more efficient and effective - Improving Japan's Participation
in Global Clinical Trials - Providing new drugs to Japanese Patients
as Early as Possible
Discussion on Advancing CTX in Japan from the Perspectives of Industry, Government, Academia, and Patients
MEMBERS
Japan CTX Research Society is composed of members from various stakeholders involved in clinical trials, including pharmaceutical companies, CROs, SMOs, IT vendors, academia, and others. Additionally, government officials and other relevant parties participate as observers (as of March 1, 2024)
- Aichi Cancer Center
- EPS Corporation
- A2 Healthcare Corporation
- Otsuka Pharmaceutical Co., Ltd.
- Okayama University Hospital
- Kindai University Hospital
- SUSMED, Inc.
- CMIC Co., Ltd.
- TechDoctor, Inc.
- Tokyo Center Clinic
- Nippon Medical School
- Eli Lilly Japan K.K.
- Pfizer R&D Japan G.K.
- Falma
- Mediford Corporation
- Merck Biopharma Co., Ltd.
- Janssen Pharmaceutical K.K.
- Linical Co., Ltd.
- Buzzreach Inc.
- IQVIA Site Solutions Japan G.K.
- Medical Research Network Japan K.K.
- MEDICOLAB Corp.
- MICIN, Inc.
Japan CTX Research Society is conducting its activities as part of the "Blockbuster TOKYO" program, Tokyo Metropolitan Government’s Startup Support Program specializing in pharmaceutical and medical startups.
Blockbuster TOKYO
Blockbuster TOKYO is a venture incubation support project in the pharmaceutical and medical field supported by the Tokyo Metropolitan Government. Based on an agreement with the Tokyo Metropolitan Government, promoters from various organizations collaborate to form an ecosystem, execute support measures, and establish an environment for creating global-level startups while focusing on brand building and effective communication. Mitsubishi Research Institute, Life Science Innovation Network Japan (Headquarters: Nihonbashi Muromachi, Chuo-ku, Tokyo; President: Hideyuki Okano), and CIC Institute (CIC Japan LLC) (Headquarters: Toranomon, Minato-ku, Tokyo; Executive Officer: Timothy Rowe) have been selected as joint promoters of Blockbuster TOKYO.
CONTACT
JCTX(Japan CTX Research Society)
Mitsubishi Research Institute, Inc,